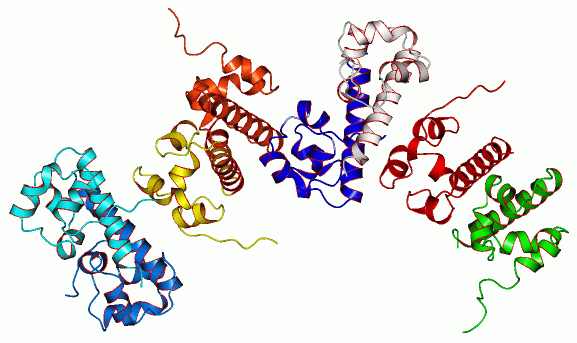

Oligomeric structure of the human EphB2 receptor SAM (sterile alpha motif) domain.
Thanos, C.D.; Goodwill, K.E.; Bowie, J.U.; Science 1999, 283(5403), 833-836 [PubMed].
The approximately 70 amino acid SAM (sterile alpha motif) domain has been identified in over 100 different proteins with diverse cellular function, from yeast to man. SAM domains have been implicated in mediating protein-protein interaction via the formation of homo and hetero-typic oligomers. The residues at the interface of the EphA4 and EphB2 SAM domain homodimers have been mapped, but the factors that determine specificity remain to be determined. The recently identified Shank family of scaffold proteins als contains a SAM domain in addition to ankyrin repeats, an SH3 (Src-homology 3) domain, a PDZ domain and a long proline-rich region
Sheng, M.; Kim, E.; J. Cell Sci. 2000, 113(Pt. 11), 1851-1856. The Shank family of scaffold proteins. [PubMed].
(Information taken in part from a compilation of protein interaction domains by T. Pawson)
More information on the structure (Protein Data Bank code 1b4f) can be obtained from the
Jena Library of Biological Macromolecules - JenaLib